As mentioned previously, heat tends to move from a high temperature region to a low temperature region. This heat transfer may occur by three mechanisms; conduction, radiation and convection. In engineering, the term convective heat transfer is used to describe the combined effects of conduction and fluid flow and is combined as the third mechanism of heat transfer.
Conduction
Conduction is the most common means of heat transfer in a solid. On a microscopic scale, conduction occurs as hot, rapidly moving or vibrating atoms and molecules interact with neighboring atoms and molecules, transferring some of their energy (heat) to these neighboring atoms.
In insulators the heat current is carried almost entirely by phonon vibrations. The “electron fluid” of a conductive metallic solid conduct nearly all of the heat current through the solid. (Phonon currents are still there but carry less than 1% of the energy.) Electrons also conduct electric current through conductive solids, and the thermal and electrical conductivities of most metals have about the same ratio. A good electrical conductor, such as copper, usually also conducts heat well. The Peltier-Seebeck effect exhibits the propensity of electrons to conduct heat through an electrically conductive solid. Thermoelectricity is caused by the relationship between electrons, heat currents and electrical currents.
Convection
Convection is usually the dominant form of heat transfer in liquids and gases. This is a term used to characterize the combined effects of conduction and fluid flow.
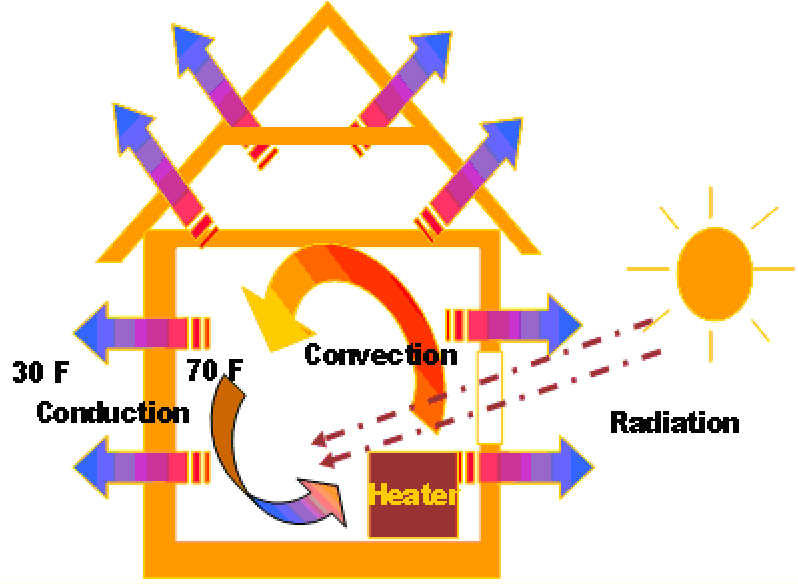
In convection, enthalpy transfer occurs by the movement of hot or cold portions of the fluid together with heat transfer by conduction. For example, when water is heated on a stove, hot water from the bottom of the pan rises, heating the water at the top of the pan. Two types of convection are commonly distinguished, free convection, in which gravity and buoyancy forces drive the fluid movement, and forced convection, where a fan, stirrer, or other means is used to move the fluid. Buoyant convection is due to the effects of gravity, and absent in microgravity environments. An example of convection is water heated up in a pot warms throughout the pot- not just the bottom.
Radiation
Radiation is the only form of heat transfer that can occur in the absence of any form of medium and as such is the only means of heat transfer through a vacuum. Thermal radiation is a direct result of the movements of atoms and molecules in a material. Since these atoms and molecules are composed of charged particles (protons and electrons), their movements result in the emission of electromagnetic radiation, which carries energy away from the surface. At the same time, the surface is constantly bombarded by radiation from the surroundings, resulting in the transfer of energy to the surface. Since the amount of emitted radiation increases with increasing temperature, a net transfer of energy from higher temperatures to lower temperatures results.
For room temperature objects (~300 K), the majority of photons emitted (and involved in radiative heat transfer) are in the infrared spectrum, but this is by no means the only frequency range involved in radiation. The frequencies emitted are partially related to black-body radiation. Hotter objects—a campfire is around 700 K, for instance—transfer heat in the visible spectrum or beyond. Whenever EM radiation is emitted and then absorbed, heat is transferred. This principle is used in microwave ovens, laser cutting, and RF hair removal.